2020 WAEC VERIFIED CHEMISTRY PRACTICAL ANSWERS
- THEORY/ESSAY ANSWERS:(1a)
Burette reading (cm)³|1st reading|2na Reading|3rd reading|
Final |15.25|30.53|45.79|
Initial |0.00|15.25|30.53|
Volume of acids used |15.25|15.28|15.26Average volume of acid used =15.25+15.26/2
=15.255cm³
=15.26cm³
Or =15.27cm³(1b)
Given; mass con of A =5g/500cm³ = 5g/0.5dn³
CA=10g/dm³
A is HNO3
Therefore molar mass =1+14+(16*3)=15+48=63g/mol¹Molarity of A = gram con/molar mass
CA=10/63=0.1587mol/dm3(1bii)
Using CAVA/CBVB =Na/NB
========================
3c (i) When exposed to light, silver chloride decomposes into gray metallic silver and chlorine. The light sensitivity of the silver chloride and other silver halides, such as silver bromide and silver iodide, forms the basis of the photographic process
========================
(3ai) Zn(NO3)2 Salt E is a Zinc Nitrate(3aii) 2Zn(NO3)2 —–> 2ZnO + 4NO2 + O2
(3c) It turns blue litmus paper red.
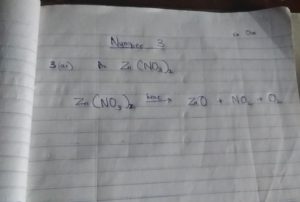
Categories: Uncategorized